|
Listen to this article  |
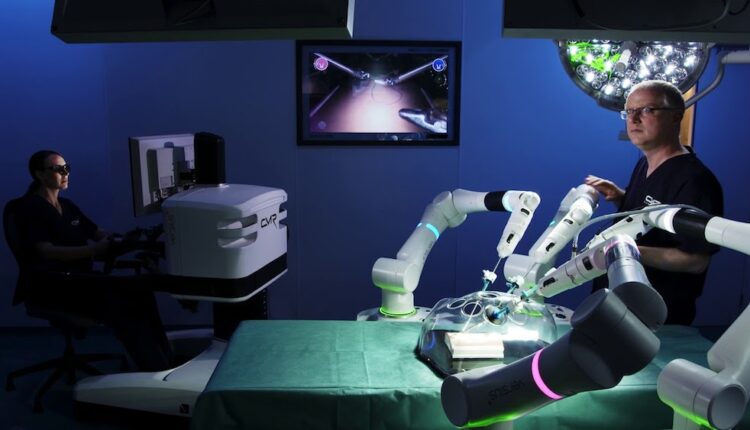
CMR Surgical today announced it has installed more than 100 Versius surgical robotic systems worldwide. Count CMR among a host of companies, big and small, that are seeking to compete against surgical robotics pioneer Intuitive.
Since introducing Versius in 2019, Cambridge, U.K.–based CMR has scaled up rapidly. There are now Versius robots in operation across Europe, Asia, Australia, Latin America, and the Middle East.
To meet the growing demand, CMR Surgical is working on building a roughly 75,000-square-foot manufacturing facility in Cambridgeshire. The new facility will serve as CMR’s global manufacturing hub. The company also has plans for additional European offices in the coming months.
CMR Surgical CEO Per Vegard Nerseth described the 100 Versius systems installed as a significant milestone for the company.
“We have seen strong and growing demand for Versius across the world in the last year as both public health systems and private centers increasingly recognize the value in a small, modular and portable system,” Nerseth said. “A key focus for us is always on finding the right partners, and we are proud to be partnering with world-class hospitals and surgeons, many of which could not previously adopt a surgical robot.”
The Versius system features freedom of port placement. The feature enables procedures tailored to the needs of each patient. Surgeons can operate the way they did laparoscopically, but with the benefits of robotic surgery, according to the company. Versius has a small, lightweight and modular design that can be moved “effortlessly.”
Health providers have used Versius in more than 5,000 clinical cases across 128 procedure types. The system has found use in specialties including gynecology, colorectal surgery, thoracic surgery, general surgery and urology.
Since it was founded in 2014, CMR Surgical has raised nearly $1 billion in funding. Its last funding round came in June 2021 when it closed a $600 million Series D round that was led by the SoftBank Vision Fund 2ii.
Editor’s Note: This article first appeared on sister publication Mass Device and was republished with permission.
Credit: Source link

Comments are closed.