|
Listen to this article  |
Noah Medical today announced positive accuracy results from a study of its Galaxy System surgical robot platform.
San Carlos, California-based Noah Medical designed Galaxy and its accessories to provide bronchoscopic visualization and access. These capabilities provide diagnostic and therapeutic procedures in patient airways.
The system features advanced imaging technologies that provide real-time location updates for potentially cancerous lesions. Noah said in a news release that it designed the technology to improve tool-in-lesion and diagnostic yield.
Galaxy received FDA clearance in March of this year. Earlier in April, Noah Medical raised $150 million to support its surgical robot platform.
The MATCH study tested the “tool-in-lesion” accuracy of the Galaxy System. Noah Medical published results for review in the Journal of Bronchology & Interventional Pulmonology.
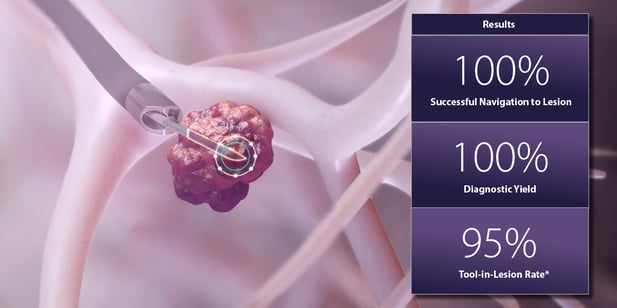
About the results from the Noah Medical MATCH study
According to a news release, the study demonstrated that the Galaxy’s TiLT Technology achieves 100% successful navigation to lesion. It also demonstrated 100% diagnostic yield and 95% tool-in-lesion accuracy. TiLT Technology features integrated tomosynthesis and augmented fluoroscopy.
“The Galaxy System was designed in collaboration with physicians, for physicians,” said Jian Zhang, Noah Medical founder and CEO. “These results help to validate the advanced design and technology of our system. This important publication comes on the heels of the recent FDA clearance of Galaxy and the first-in-human trial now underway in Australia. The Galaxy System is on a rapid path to commercialization in pursuit of our mission to enhance the quality of life for patients globally.”
MATCH aimed to assess the tool-in-lesion accuracy in peripheral lung nodules, confirmed by CBCT in a porcine model. Noah said the results offer critical insights because current robotic platforms remain prone to CT-to-body divergence. The company said confirming a high rate of tool-in-lesion could enable Galaxy to increase the rate of definitive diagnosis.
Principal investigator Dr. Krish Bhadra called Galaxy “a new category of image-guided robotics.” Bhadra, out of CHI Memorial Hospital in Chattanooga, Tennessee, said the study “gets us one step closer” to the goal of solving CT-to-body divergence in interventional pulmonology.
Editor’s Note: This article was republished from MassDevice, a sister publication of The Robot Report.
Credit: Source link

Comments are closed.