Pioneering a more ethical approach to clinical trials on animals and humans, Brisbane-based biotech startup Gelomics is having a life-changing impact in more than 21 countries. Being a part of Trade & Investment Queensland’s Go Global Export Program has been instrumental to their exporting success.
When we hear about clinical trials, it’s often about the results of a study and not the ‘how’.
The facts of the ‘how’ are sobering. More than 200 million animals are euthanised for research and development every year. Over 90 per cent of drug candidates in human trials fail, despite animal testing. It takes an average of 10 to 15 years to develop a new drug, and the cost to develop a drug is around US$2.6 billion.
Dr Christoph Meinert lives and breathes the ‘how’. Over the past 15 years, the researcher-turned-entrepreneur has worked in some of the world’s most prestigious labs and seen firsthand the barriers to developing lifesaving treatments every single day.
“There’s literally millions of cell and animal experiments performed every single year globally in academic and pharmaceutical laboratories,” Christoph tells Startup Daily. “But only a very minuscule proportion of the data that’s produced using such experiments actually predicts how human tissues and organs would react, for example, to drug candidates.”
While it’s been a well-known issue in the life science industry for a long time, the technology hasn’t been available to do something about it. Until now.
Evolution of a cell revolution
While studying a PhD in Bioengineering at the Queensland University of Technology, Christoph came up with a solution.
In 2021, Gelomics launched its flagship product, the LunaGel™ 3D Tissue Culture System, which allows human cells to develop into microscopic representations of human tissue in a petri dish.
Traditionally, cells are grown and tested in a two-dimensional plastic petri dish, which is a “highly unnatural” environment that leads to misleading data, says Christoph.
The results are life-or-death: animal and human lives are lost due to faulty and unsafe drugs. So labs using Gelomics can undertake animal-free research that’s more accurate and cost-effective, as well as being safer and faster in identifying unsafe or ineffective drugs before they make it to trials in areas like cancer treatment.
“Our 3D tissue culture approach represents a transformation to drug development and has wide ranging implications for more human and animal lives,” Christoph explains.
“It’s really a game changer. Scientists can now grow tissues that are going to not only replicate a real thing, but also look, feel, and behave like it. So you can imagine tiny, beating little heart muscles or intricate vascular systems, functional livers or even microscopic little tumours that can be used for drug development.”
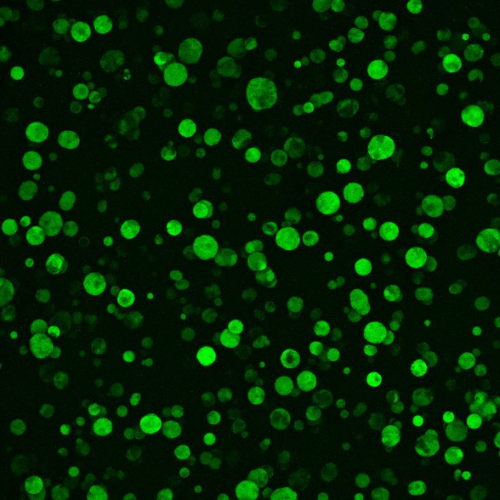
Breast cancer tumour organoids grown in Gelomics’ product. Image: Supplied.
Exporting Gelomics to the world
Exporting the innovation was going to be key to Gelomics’ success. Like many first-time founders, Christoph went on a steep learning curve.
“Exporting was entirely uncharted waters for us,” he tells us. “We knew that with the solution we have, we have to go global to truly have an impact on the industry. So we embarked on this journey learning about international regulations, cultural business nuances, and also the importance of local partnerships through distributors that we had as well.”
Trade & Investment Queensland (TIQ) proved to be a transformative partner in Gelomics’ exporting journey. Gelomics applied for TIQ’s Go Global Export Program, which gives export-ready Queensland businesses financial and on-the-ground support to finalise an export deal in a new market.
The program provides funding to help small and medium-sized Queensland businesses overcome specific challenges they are facing with a planned export transaction into a new international market, through matched funding up to $25,000 (excluding GST).
Upon receiving the grant, Gelomics were able to make inroads in Japan and get their first clients.
“The grant provided us with invaluable introductions, mentorship and on-site support,” Christoph says. “It was good to have somebody there to train us and mentor us throughout the process as well. So it wasn’t just about the funding, but it was also about bridging these cultural and business divides, which was establishing trust and laying a foundation for a partnership.”
Christoph says the grant helped kicked off what is now a “very active export program” with Japan. It also gave them the chance to provide sales and marketing training on the ground and get to know their distributor and key opinion leaders in the scientific fields they were servicing.
Since then, more countries have taken steps towards eliminating animal testing, which is only good news for Gelomics. Today, their 3D tissue culture models are being exported to more than 200 labs across the Asia-Pacific, Europe and USA.
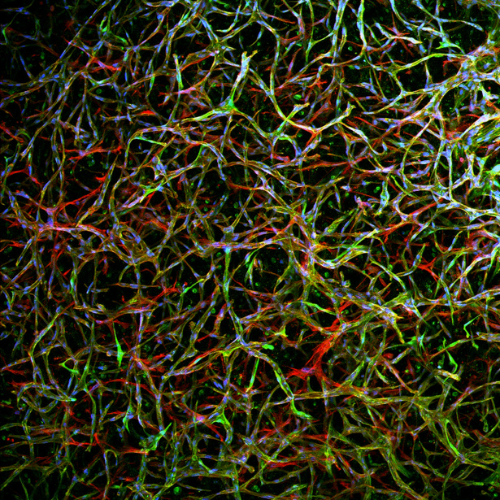
Vascular tissue grown in Gelomics’ product. Image: Supplied.
Apply for the Go Global Export Program’s 2023 grants
Christoph recommends other export-ready Queensland-based businesses seize the opportunity now before applications for this year’s program close on September 11, 2023.
“My advice would be to embrace the opportunity,” he says. “The program is not just a grant. It’s really a partnership. It offers businesses a wealth of resources, networks and expertise that can be really instrumental in navigating complexities of new international geographical markets, which are often challenging to navigate initially.”
The Go Global Export Program is currently looking for innovative businesses across a diverse range of industries. Are you the next Gelomics?
Apply for the Go Global Export Program now for your chance to receive a $25,000 grant and support from Trade & Investment Queensland to help you export to a new market.
This article is brought to you by Startup Daily in partnership with Trade & Investment Queensland.
Credit: Source link


Comments are closed.